1. What’s electrolysis really for?
Think about the copper in your phone or laptop – it doesn’t come out of the ground ready to use. After smelting, you’re left with rough, dark copper slabs packed with impurities like gold, silver, and lead. Electrolysis is the process that turns this “messy mix” into “pure water.”
In simple terms, it cleans up crude copper and gives old wires and scrap a “second life,” turning them back into usable copper. Without this step, a lot of today’s tech probably wouldn’t even work.
2. How does this “copper wash” actually work?
It’s easier than it sounds – imagine copper moving house.
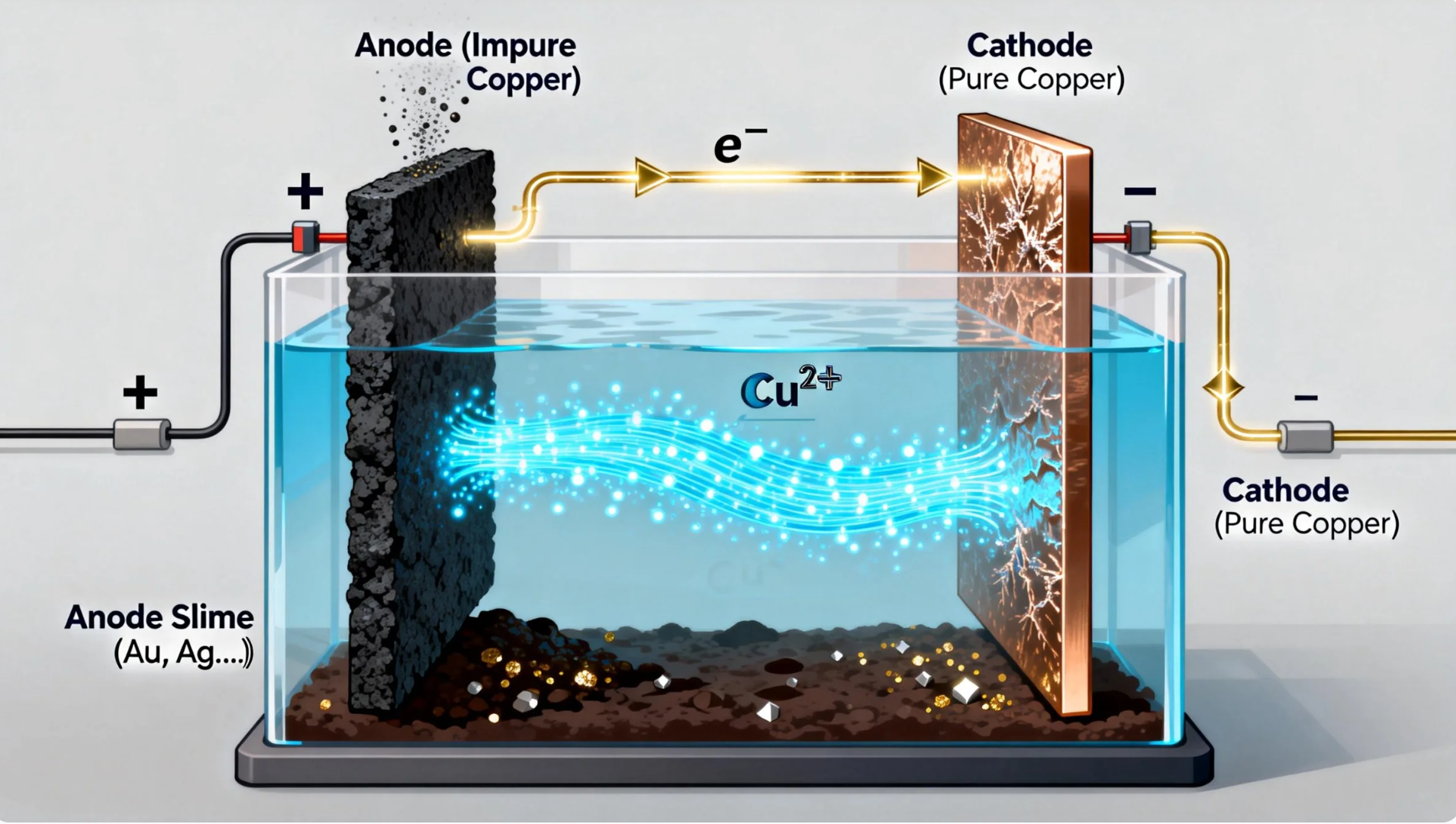
You’ve got a large tank filled with a copper sulfate solution, almost like a little swimming pool. Hang a dirty, thick copper plate (the anode) on one side and a bright, thin pure copper sheet (the cathode) on the other.
When you switch on the power, the dirty plate gradually dissolves into copper ions, which get “pulled” toward the pure copper sheet, where they settle back into solid copper, building up layer by layer in an orderly way.
The impurities don’t tag along: gold and silver sink to the bottom as black sludge (anode slime), which can be collected separately. Other impurities either dissolve in the liquid or settle at the bottom. After the move, what’s left is the purest copper.
3. Why go through all this trouble?
Mainly for three reasons: purity, profit, and the planet. Purity: Electrolytic copper can reach over 99.99% purity. When you’re making chips or precision instruments, even a tiny impurity can ruin everything. Profit: The anode sludge contains recoverable gold and silver – basically like finding “buried treasure.” Planet: Paired with modern wastewater treatment systems, the whole process can be kept clean and environmentally controlled. For recyclers, this is the most cost-effective way to turn scrap copper into something as good as “gold.”
4. The key to electrolysis – steady current
The real hero here isn’t the tank – it’s the electricity.
If the current fluctuates up and down, the copper plating ends up uneven and pitted, looking like it’s been chewed on, and it wastes both material and energy. So the current needs to be as steady as a straight line.
In the factory, this job falls to the rectifier cabinet. It can handle tens of thousands of amps, with a casing that resists acidic fumes and an internal system that smartly regulates temperature and prevents overheating. If this thing fails, the whole production line grinds to a halt.
5. Where is this used?
Pretty much everywhere:
- Large smelters: Turning mined copper into high-purity copper.
- E-waste recyclers: Recovering copper from old computer motherboards.
- Copper foil plants: Making the thin foil used in smartphone circuit boards.
- All kinds of metal recovery facilities: Extracting copper from industrial waste.
In short, if the goal is to get copper clean – whether it’s fresh from the mine or recycled from scrap – it pretty much has to go through electrolysis. It’s the unsung hero of modern industry, and it’s what makes the idea of a circular economy more than just talk.
Post time: Jan-09-2026